Executive Summary
- AlphaFold, developed by Alphabet’s DeepMind, and its discovery of hundreds of thousands of new psychedelic molecules that could lead to new antidepressants, highlights a growing trend of artificial intelligence (AI) adoption in the pharmaceutical industry (pharma).
- The White House’s Executive Order on AI produced October 30, 2023, has charged regulatory agencies from the Department of Health and Human Services (HHS) to the Federal Drug Administration (FDA) to quickly roll out safety standards and measures surrounding the use of AI in pharma (AI pharma).
- Adopting sensible regulations for AI drug development and ensuring consumers healthcare data is used with their privacy in mind, are actions that government agencies and pharma companies must tackle soon.
Introduction
Despite funds being poured into the pharma industry for decades, it was not until the past several years that major breakthroughs in discoveries, approval of certain technology’s use, and AI’s rapid development in creating drugs did AI in medicine begin to take form. The Human Genome Project’s founding in 2003 opened the door to mass amounts of data for potential use in AI and deep learning (DL) tools to be utilized in medicine; but it was not until IBM’s debut of Watson in 2007, founding of companies like Exscientia in 2012, and the FDA’s first approval of a clinical cloud-based DL application in healthcare in 2017 that serious investigation into AI’s potential uses began to take hold.
Over the past decade, these rapid advancements in AI-based tools has allowed pharma developers to more rapidly and accurately than ever discover new medicines and applications. The World Health Organization published a set of considerations for regulating AI in healthcare in October 2023, that largely focused on the current industry landscape rather than prescribing recommendations. But outside vague attempts at proposed policy frameworks for regulating the area by the FDA in 2019, 2021, and again in 2023, no serious call to action has been taken to dictate boundaries for AI pharma. The FDA has neglected to provide explicit guidance to time-sensitive questions on AI pharma, opting solely to provide a list of authorized AI-enabled medical devices.
The lack of oversight in an industry that necessitates it, principally to ensure trust and provide ethical care to consumers, with a technology that presents more problems than any other in the history of healthcare, is concerning. Moving forward, agencies and regulation should be targeted and explicit and take a more stringent approach to AI than will be taken in other industries, as missteps in AI pharma could pose irrevocable harm to consumers.
Overview of AI Pharma Today
Investment towards AI pharma is projected to double over the next three years and more than 10x in the next decade. Drugs with capabilities only accessible through AI and DL are able to be created, all while the cost to develop them drops to 70%. While cash pours in to help build and share in the profits of these technologies, regulatory uncertainty and surrounding potential harms could lead investors walking away from AI pharma and adjacent technologies.
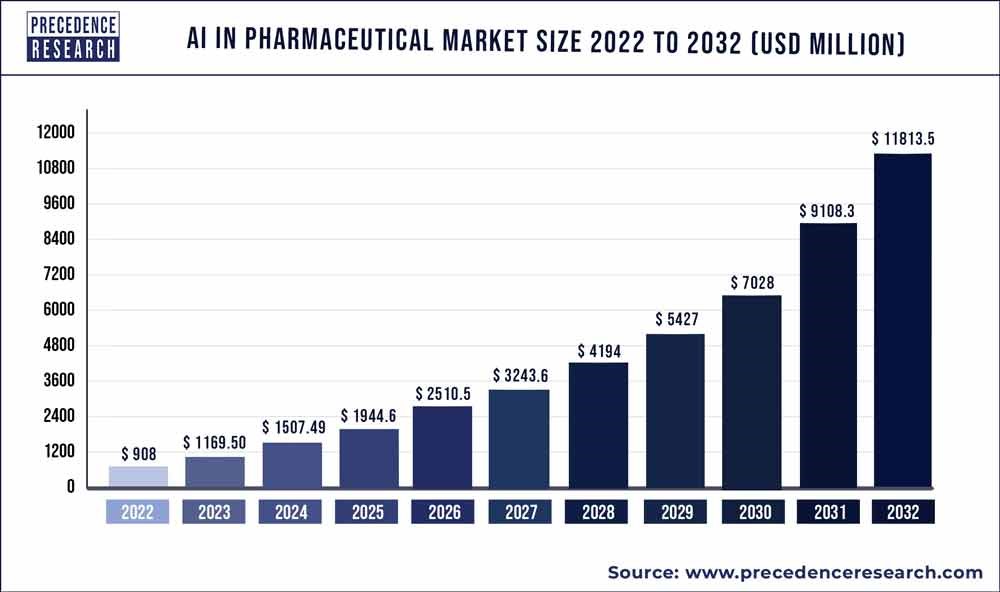
AI pharma presents potentially its most extensive benefits in leveraging large datasets, identifying patterns, increasing accuracy and being more cost and time efficient. Several pharma companies have drugs in or on the cusp of clinical trials that were created primarily through AI and DL tools. DL can help develop the drugs faster with deep sets of data, scanning billions of public health records and private data sets allow companies to identify clinical patients, and AI can then aid further in the clinical development stage. From 2016 to 2022 the FDA received over 300 applications for AI drug development, with the vast majority coming in the last two years of that period. As AlphaFold, a tool developed by Google’s DeepMind, has demonstrated through experimentally derived protein structures using an AI tool (which can be discovered in minutes rather then years), BigTech and AI-focused startups are showcasing that advanced AI and DL give rise for technology-focused organizations, as opposed to pharma-centric startups, to be legitimate players in the industry.
Major Questions Surrounding AI Pharma
Because many of these AI tools are using first-of-their-kind methods and utilize human data and models in the pre-trial phases, unlike historical animal testing, the more AI is leaned on in drug development, the more unanswered nuances that AI pharma brings become present. Regulations are sparse and agencies are silent on fundamental questions regarding drugs developed in large part by AI:
- What does adequate testing for drugs developed by AI Pharma look like?
- What types of evidence needs to be in a submission filing?
- What level of human intervention is necessary at each stage of development to upend shortcomings in AI?
Until these and many more questions are answered by governing bodies approval of these drugs will be near impossible. If these questions continue to go unanswered, advancement and adoption of AI pharma will slow down. While the problems surrounding its implementation are abundant, the benefits of AI pharma development are undeniable and present too much upside for regulations to stall its evolution.
The lack of access to large swaths of high-quality data, needed for AI pharma to function properly, for smaller and newer pharma companies poses a threat to further isolate an already oligopolistic market for drugs even further. While AlphaFold is a good example of a non-pharma giant discovering a new protein structure tool through the use of proprietary AI, most production and distribution channels are by a wide margin controlled by legacy pharma companies. From the consumer point of view, the results of this are twofold. On one hand, without further input from governing bodies on how this industry can operate with AI, Americans may continue to fall victim to oligopolistic pricing schemes run by a few dominant players who utilize health data without direct knowledge for the everyday consumer. On another, economies of scale in the industry can be viewed as an efficient angle for AI pharma giants, with the most sophisticated and well positioned companies channeling most of the funds and talent to make AI pharma reach its potential and get lifesaving drugs out quicker. A discussion of proper guardrails for the amounts and types of health data that AI pharma developers can access is of high order to protect consumer privacy.
For AI pharma to be productive, the data necessary for it to be useful likely includes patient health information to create new drugs. Large health systems and AI developers have shared patient information in a lucrative endeavor, and AI pharma has the potential to predict identifying information about consumers that was not known prior. While this insight about AI’s data usage with medicine may lend a familiar eye to Moore v. Regents of the University of California, but the use of medical information in AI for the purpose of creating new drugs is a distinct and discovering specific insights about consumers is an unexplored area unlikely to draw serious comparison to any case previously. If for nothing else, the sheer scale of some of the datasets required for DL tools in AI pharma pose privacy issues never seen before in medicine. As the FDA, HHS, and other bodies begin to release concrete regulations regarding AI pharma, keeping in mind the privacy of every person’s own health and health data must be a principal concern. Without doing so, the consequences for the public are vast; perhaps none more than a continued general distrust in the pharma industry. Setting standards for how healthcare data is shared and used among AI pharma developers can help ensure that consumer privacy remains a focus in the space.
Conclusion
As AI pharma continues to progress in both the private and public spheres, it is imperative that government agencies, Congress, and other regulators begin to step in with sophisticated and detailed guidance on how drug development can and should progress with AI in the mix. To best advance AI pharma ethically and efficiently and protect consumer welfare, regulations regarding AI-developed drug progression must incorporate the same scrutiny that longstanding regulations for non-AI-developed drugs must stand to.
You must be logged in to post a comment.